Written on: April 6, 2021 by Dr. Paul Jackson
In many parts of the world, concerns are being raised about the inhalation of volatile chemicals and fine particles. Spray products, and aerosols in particular, are susceptible to these concerns because they both use volatile chemicals and put fine particles into the air. However, in many countries, the regulatory frameworks that exist to place aerosols on the market have already addressed these concerns. For example, in Europe, aerosol dispenser regulations require the marketer of an aerosol to conduct hazard assessments that include consideration of the risk from inhalation of a spray.
In 2013, the British Aerosol Manufacturers’ Association (BAMA), working with sister associations in the EU for aerosols, cosmetics and detergents, and with the help of the Research Institute for Fragrance Materials (RIFM), produced a guide on safety assessment. It was published the following year in the journal Toxicology Letters as an article titled “Principle Considerations for the Risk Assessment of Sprayed Consumer Products” (Toxicology Letters 227 (2014) 41-49).
On Feb. 24, 2021, at the invitation of the Household & Consumer Products Association (HCPA), I presented to HCPA and National Aerosol Association (NAA) members, via webinar, the five-step methodology that BAMA has developed from these principles to enable aerosol marketers to assess product inhalation risk during consumer use. The BAMA Methodology is represented in Figure 1.
The BAMA Methodology uses guidance that has been published to support EU regulations on the use of chemicals and cosmetic products, but is tailored to be accessible to small- and medium-sized aerosol companies. Key to the Methodology is to only complete those stages needed to show that the aerosol is safe to use.
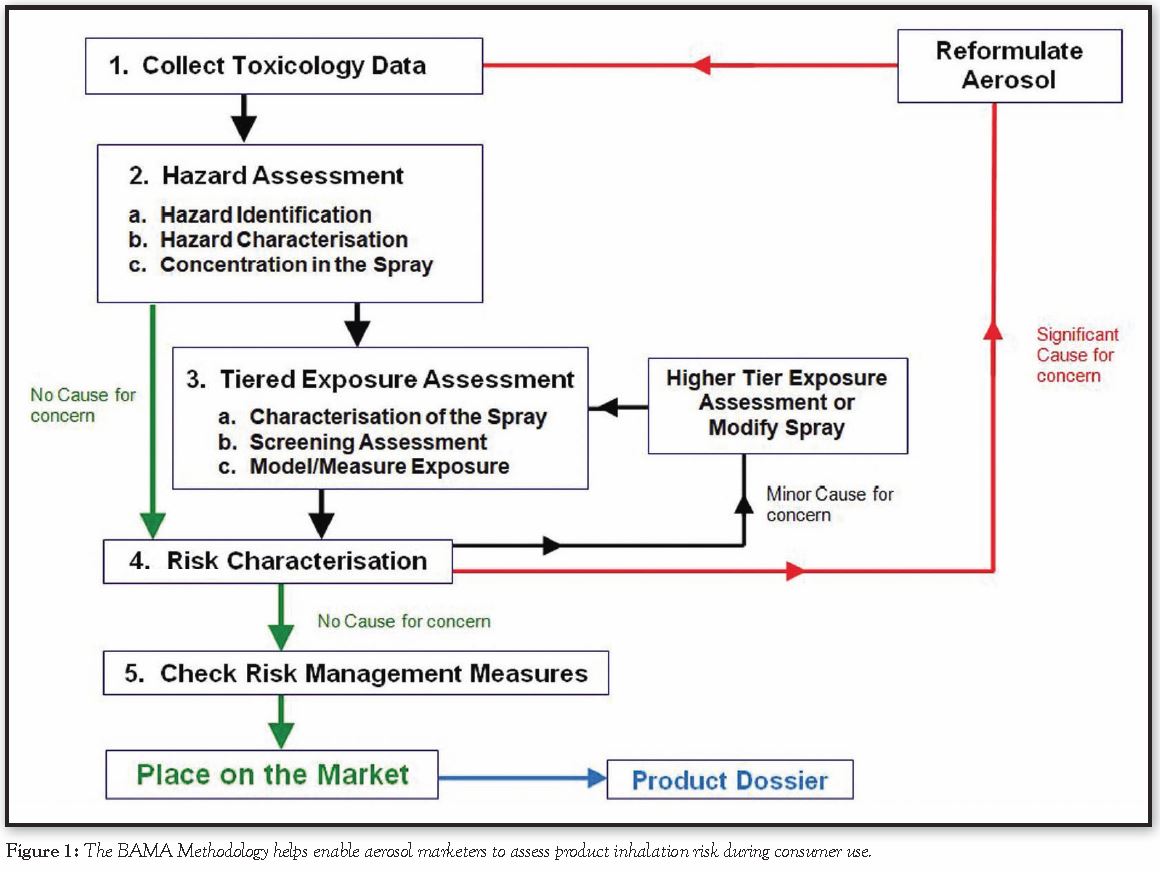
To explain how to do this, BAMA has developed a detailed guidance, summarized as follows:
Step 1: Collect Toxicology Data
The ingredients used in the aerosol formulation will have safety data sheets (SDS) if they have any hazardous properties. If the ingredients used are not hazardous, then the aerosol spray is unlikely to be hazardous, and the risk assessment is complete.
Step 2: Assess the Hazards Present
This consists of three tasks: identifying the hazards, identifying the concentration that does not present a hazard to the user and checking if the ingredients are used at lower concentrations. Step 2 may rule out the use of some ingredients.
Step 3: Assess the Exposure When Using the Aerosol
This requires a tiered approach that involves answering the questions How is the aerosol dispensed? and What would be the exposure to the person using the product? For Step 3, there is the opportunity to model or measure the exposure from using the aerosols. Modeling is easier but, depending on the sophistication of the models, will need varying amounts of input information and the output exposures will usually be an overestimate of the real exposure. Models range from the relatively simple and easy-to-use BAMA Indoor Air Model to some quite sophisticated models based on computational fluid dynamics that give more accurate exposure estimates but require expertise to use. Measurement can be the most accurate way to estimate exposure, but it is expensive and needs expertise to ensure that the data measured is relevant to the use of the aerosol.
Step 4: Characterize the Risk of Using the Aerosol
Once the exposure during aerosol use has been estimated, modeled or measured, the next step is to assess the risk to human health of that particular level of exposure compared to the regulatory limits. In Europe, for aerosols used in industrial or household settings, this is done by calculating the risk characterization ratio (RCR), while for cosmetic products, it is done by calculating the margin of safety (MoS). Both are similar approaches but come up with slightly different numbers.
Step 5: Check the Correct Risk Management Measures are in Place
Step 5 should be very familiar because it is the process that most companies undertake prior to placing a product on the market. Be sure to check that the aerosol has the correct labeling and safety features.
As mentioned above, the key to BAMA’s Methodology for Inhalation Risk Assessment is to only do the steps necessary to show that the aerosol is safe to use. Sometimes this may mean that certain ingredients cannot be used; in other cases it may mean that changes to the valve or propellant are needed in order to increase the particle size. However, aerosol products, when formulated correctly and with the exposure risk properly assessed, do not present any greater hazard to users than any other product. This is probably why over 15 billion aerosols are sold around the world each year.
For more information on BAMA’s Methodology for Inhalation Risk Assessment, please contact the BAMA office at enquiries@bama.co.uk. SPRAY