Written on: June 1, 2021 by Cassandra Taylor
If you sell hazardous products in Europe or the United Kingdom (UK), you are probably already aware that the chemical regulations are changing. On Jan. 1, 2021, the UK became a “third country” to the European Union (EU). This article will review some recent updates to the EU and UK hazardous materials regulations, and how they relate to each other in the aftermath of Brexit.

EU SDS updates (REACH Annex II)
In July 2020, Annex II of the Registration, Evaluation & Authorization of Chemicals (REACH) Regulation (EC) No 1907/2006 was amended. The new amendment, Commission Regulation (EU) 2020/878, came into force on Jan. 1, 2021. REACH Annex II describes the requirements for the compilation of Safety Data Sheets (SDS), including layout and content.

Amendments to Annex II were necessary to incorporate specific EU requirements for nanoform substances, endocrine disruptors and unique formula identifiers (UFI) into the appropriate subsections of the SDS.
The updated REACH Annex II addresses the following:
• UFI (section 1.1, when it is indicated on the SDS)
• Nanoform properties (sections 1, 3 and 9)
• Endocrine disruptors (sections 2, 11 and 12)
• Additional information for substances included in Part 3 of Annex VI to Regulation (EC) No 1272/2008 (section 3, when available):
• Specific concentration limits (SCLs),
• M-factors,
• Acute toxicity estimate (ATE)
• Section 3 disclosure limits
• Closer alignment with the Globally Harmonized System of Classification & Labeling of Chemicals (GHS) 6th and 7th edition

Among the disclosure changes, skin and respiratory sensitizer 1A chemicals will now have to be disclosed when present at 0.01% or 1/10th of the assigned SCL. Substances that are classified for aspiration toxicity must be disclosed when in the mixture at 1% or more, rather than 10% as previously indicated.
Great Britain (GB) has not officially adopted the Annex II update. This means that there are now differences in the format and content provisions laid out for GB versus EU SDS. As a result of the Annex II amendments, EU SDS are expected to be more comprehensive compared to the current GB and previous European SDS requirements, with additional substance and product data points needed for compliance.
A grace period of until Dec. 31, 2022 has been provided to update all current EU SDS to conform with the new requirements.
EU CLP updates: ATP 14
The 14th Adaptation to Technical Progress (ATP) of the EU Regulation (EC) No 1272/2008 on the classification, labeling and packaging of substances and mixtures (CLP) becomes mandatory on the date of application: Sept. 9, 2021.
ATP 14 includes new classification and labeling requirements for products containing titanium dioxide:
• Liquid products containing 1% or more of Titanium dioxide particles with aerodynamic diameter equal to or below 10μm will need to be classified as Carcinogen 2 and labeled with the EUH211 statement: Warning! Hazardous respirable drop lets may be formed when sprayed. Do not breathe spray or mist.
• Solid mixtures containing 1% or more of Titanium dioxide will need to be will need to be classified as Carcinogen 2 and labeled with the EUH212 statement: Warning! Hazardous respirable dust may be formed when used. Do not breathe dust.
Table 3 of Annex VI to CLP has been amended to include three new notes that relate to the changes for Titanium dioxide. Note V addresses Titanium dioxide fibers and sanctions the assignment of a more severe category (Carc. 1B or 1A) and/or additional routes of exposure when appropriate. Note W describes the particular toxicity of Titanium dioxide as it relates to respirable dust. Note 10 specifies that the carcinogen by inhalation classification applies only to mixtures containing 1% or more of Titanium dioxide in the form of particles with an aerodynamic diameter ≤ 10μm.
Other changes to Annex VI under the 14th ATP of CLP include two deleted substances, 12 updated substances and 17 new substances. Ten new ATEs have been specified in the update as the European Chemicals Agency (ECHA) moves toward inclusion of this relatively new-to-Annex VI data point.
If you are using or supplying substances listed in the ATP 14 update to Annex VI, you will need to re-classify your products and update your SDS based on the new harmonized substance requirements before the date of application.
ATP 14 was adopted before the UK became a third country. Therefore, the new classification and labeling information for Titanium dioxide and the other updated chemicals are also appearing on the GB mandatory classification and labelling list (GB MCL) and must be used for SDS and label generation.
Other ATPs to EU CLP
Several other CLP ATPs are also currently in the works:
• ATP 15 applies from March 1, 2022. Changes to Annex VI of CLP include 37 new entries, 21 revised entries and two deleted entries.
• ATP 16 is currently in draft form. This ATP concerns 15 substances that can be used in cosmetics.
• ATP 17 is currently in draft form. Changes to Annex VI of CLP include 22 new entries, 41 revised entries and one deleted entry. Publication in EU Official Journal is expected soon, with an anticipated application date in mid-2022.
• ATP 18 is currently in draft form. Changes to Annex VI of CLP include 36 new entries and 20 revised entries.
Brexit: EU (Withdrawal) Act 2018 (2020)
Since Brexit, the UK has taken a “lift and shift” approach for all EU chemicals management regulations. The EU (Withdrawal) Act 2018 (2020) converts directly applicable EU law into domestic law in GB. This means that each EU regulation as of Dec. 31, 2020 has been transposed into UK regulation and is applicable in GB from Jan. 1, 2021. For the time being, British legislation remains almost completely aligned with EU legislation. EU regulations implemented this year, such as Regulation 2020/878/EU discussed above, are the exceptions to this rule.
New British regulations transposed from EU regulations include:
• CLP -> GB CLP
• REACH -> UK REACH
• PIC -> GB PIC
• BPR -> GB BPR
• PPPR -> GB PPPR
• SEVESO -> GB SEVESO
• Detergents -> GB Detergents
GB did not directly adopt Annex VI of CLP regulation. The MCL was implemented, which currently matches the harmonized classification and labeling entries found in Annex VI. As GB begins to implement its own requirements, we anticipate that the MCL will begin to deviate from the EU CLP classification requirements.
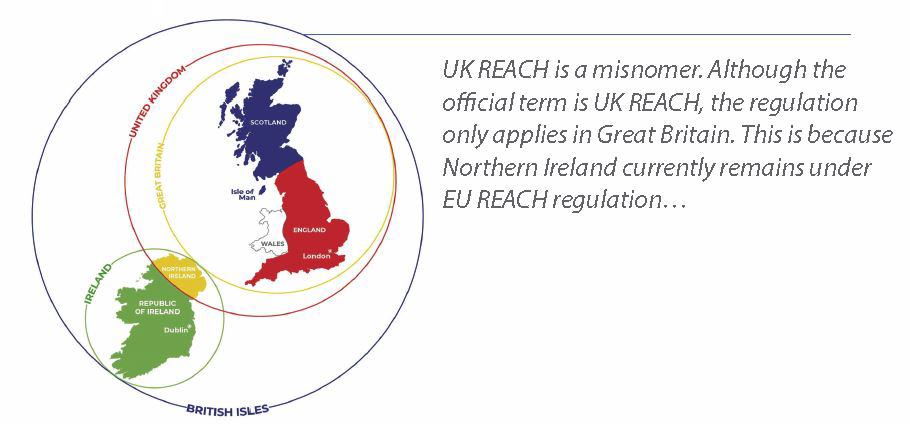
You might have noticed that UK REACH stands out from the other regulations listed above. Therefore, is it UK REACH or GB REACH? UK REACH is a misnomer. Although the official term is UK REACH, the regulation only applies in GB. This is because Northern Ireland (NI) currently remains under EU REACH regulation.
Due to its geographical location, it is desirable to maintain an open border between the Republic of Ireland (ROI) and NI, even though NI is part of the UK. The Protocol on Ireland/Northern Ireland was established so that NI would remain aligned to EU regulations rather than GB regulations. This means that NI is still subject to EU CLP, EU REACH and so on. The protocol is subject to review every four years.
The Health & Safety Executive (HSE) is the British agency that is taking the place of ECHA in the UK. The HSE is the designated authority responsible for implementing and enforcing GB chemicals management regulations.
In GB, the National Poisons Information Service (NPIS) is the appointed body responsible for receiving specific information from GB importers and suppliers about the ingredients of hazardous mixtures that they place on the GB market. The current scheme of data collection is voluntary and there is no legal obligation to notify NPIS in GB.
The main takeaway is that British hazardous chemicals regulations are still largely the same as the corresponding EU regulations, with technical amendments applied to make them relevant to GB rather than the EU. As we move forward, we can expect to see more divergence as GB shifts towards its own individualized chemicals management scheme. We will be sure to keep you informed on European and British hazardous chemicals regulations as new requirements are implemented. SPRAY