Written on: September 1, 2023 by Cassandra Taylor
There is a lot to report on in the sphere of hazardous materials regulations these days and this seemed like an excellent opportunity to do a worldwide update to keep SPRAY readers informed of changes happening in some key global jurisdictions.
Brazil adopts GHS 7th Revised Edition
On July 3, the Brazilian National Standards Organization (ABNT) adopted a long-awaited revision to the Brazilian Globally Harmonized System of Classification & Labeling of Chemicals (GHS) standard NBR 14725, thereby implementing the 7th Revision of GHS.

Since Brazil is still using GHS Revision 3, this means that the update will include changes to the Aerosol Hazard classifications. “Flammable Aerosols” Categories 1 & 2 will now be “Aerosols–Category 1” and “Aerosols–Category 2”. Additionally, the Aerosol 3 category will be added for non-flammable aerosols. The new “Chemicals Under Pressure” classification will also be adopted.
New hazard classifications and other provisions from GHS Revision 7 will also be implemented with this update. Another notable change will be to the acronym used for safety data sheets (SDS) in the Portuguese language. Currently, the Portuguese acronym “FISPQ” is used, which originates from the European language standing for Ficha de Informações de Segurança de Produtos Químicos. The new acronym for SDS using the Brazilian Portuguese language will be “FDS” for Ficha com Dados de Segurança.
UK to ease GB chemical industry regulatory burden
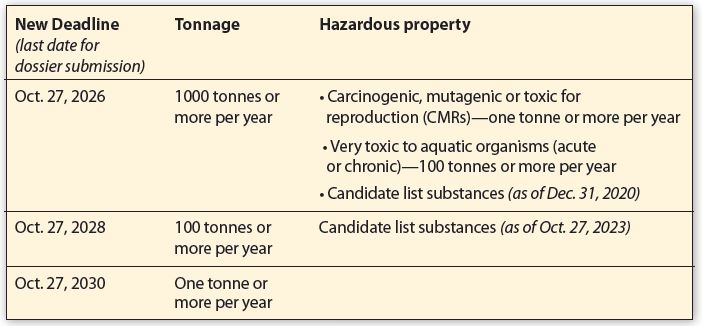
The United Kingdom’s (UK) Dept. for Environment, Food & Rural Affairs (DEFRA) has signed into law long-awaited extensions to UK Registration, Evaluation, Authorization & Restriction of Chemicals (UK REACH) registration deadlines. With the official release of REACH (Amendment) Regulations 2023, companies will have three additional years to submit a full dossier to the Health & Safety Executive (HSE). DEFRA has stated it is committed to exploring the development of an alternative model for transitional registrations to ease the burden on Great Britain’s (GB) chemical industry. The updated registration deadlines are based on tonnage as well as hazardous properties and they can be found in the chart below.

The UK Dept. of Health & Social Care (DHSC) recently announced that Annex VIII of Regulation (EC) No 1272/2008 on Classification, Labeling & Packaging (CLP) will be revoked by the end of 2023. Annex VIII, which was inadvertently adopted by the DHSC after Brexit, concerns harmonized information relating to emergency health responses. The change means that GB companies will no longer be required to submit a poison center notification in the harmonized format to the National Poisons Information Service (NPIS). GB companies will need only to submit a voluntary notification by emailing a copy of the SDS to the NPIS.
In the meantime, HSE is expected to continue to maintain a pragmatic and proportionate response to enforcement activities, basing decisions on the level of risk and the unique circumstances of the compliance concern. For the Northern Ireland market, the Annex VIII notification requirements will remain in place.
EU proposals to amend detergents regulation
On April 28, the European Commission published a proposal on detergents and surfactants, amending Regulation (EU) 2019/1020, and repealing Regulation (EC) No 648/2004. The draft regulation would repeal the 2004 detergents regulation and replace it with a modernized version that contains new requirements for anyone manufacturing, importing or supplying detergents in the European Union (EU).
The updated regulation would remove the obligation for an ingredient data sheet for hazardous detergents, replacing it with a new Product Passport requirement. The Product Passport will contain a unique identifier connected to the detergent that will be included in a central European Commission registry set up under the Ecodesign for Sustainable Products Regulation1.

The definition of detergent would be updated to incorporate new products, such as those containing microorganisms. In addition to new provisions for the use of microbes, the revision would also establish rules for refillable detergents and digital labeling. Finally, the changes would address the overlap in requirements to comply with current legislation governing detergents, and requirements set out in the CLP, Biocidal Products and REACH Regulations, specifically when it comes to labeling.
The comment period for the proposal, which is still in draft form, ended in June. The proposed transition period to bring detergents into compliance is 30 months once the new regulation enters into force.
Singapore adopts GHS 7th Revised Edition
Revised GHS standards SS 586–2022 Series: Specification for Hazard Communication For Hazardous Chemicals & Dangerous Goods were released by the Singapore Standards Council on Feb. 6, 2023. Part 2 of the series covers Singapore’s adaptation of GHS. Part 3 covers SDS preparation.

The updated standards implement Revision 7 of the GHS. There is a 24-month transition period during which either the 2014 version according to GHS Revision 4 or the updated 2022 standard may be followed in the meantime. From Feb. 6, 2025, hazardous products must be classified according to the updated GHS Revision 7 Singapore requirements.
A new physical hazard—desensitized explosives—has been added, along with new sub-categories for Flammable Gas Category 1 (1A and 1B) and Pyrophoric Gas. When it comes to the Singapore SDS, there are new provisions for identification of nanomaterials, as well as a guide for selecting the masked name and generic name for chemicals that are confidential business information (CBI). SDSs must be reviewed every five years.
Malaysia proposes to adopt GHS 8th Revised Edition
The Malaysia Dept. of Occupational Safety & Health (DOSH) has proposed moving from GHS Revision 3 to Revision 8. The update to the Occupational Safety & Health (Classification, Labeling & Safety Data Sheets of Hazardous Chemicals) Regulations 2013 (CLASS Regulations 2013) is predicted to be finalized some time in 2023. It is not clear what the transition period will be for implementation.
Like Brazil, Malaysia is currently using GHS Revision 3, which means that the aerosol classifications will need to be updated. The Skin Irritation 3 building block, which was previously excluded in Malaysia GHS, will now be adopted. Additionally, sub-categories for Eye Irritation 2A and 2B, as well as Respiratory/Skin Sensitization 1A and 1B, have been added.

We are also anticipating an update to Malaysia’s GHS classification list, Industry Code of Practice on Chemicals Classification & Hazard Communication (ICOP). ICOP was most recently updated in 2019 and currently includes mandatory classifications for 662 specified substances.
Health Canada ends animal testing
The Government of Canada recently passed Bill C-47, Budget Implementation Act, 2023, No. 1, which amends the Food & Drugs Act (FDA) in Canada to ban testing of cosmetic products on animals. The legislative update means that as of Dec. 22, 2023, companies will no longer be allowed to test cosmetic products on animals or sell cosmetics that rely on animal testing data to establish safety.
Canada will join the ranks of other global leaders that have already taken steps to ensure ethical cosmetic testing, including Australia, UK, South Korea and all EU countries. Health Canada is working with international committees and organizations such as Economic Co-operation & Development (OECD) and the International Cooperation on Alternative Test Methods (ICATM) to develop, validate and implement effective alternatives to animal testing.

Additionally, Health Canada has launched a new quarterly Workplace Hazardous Products Program (WHPP) newsletter2 to help stakeholders stay up to date with the latest news related to the Hazardous Products Act (HPA),the Hazardous Materials Information Review Act (HMIRA) and their regulations.
The latest newsletter, released in June, includes updates about the new label compliance tool3 on WHMIS.org and a summary of WHPP’s engagement activities for the previous quarter. Health Canada also released a summary of questions and answers from the February 2023 webinars and the May 2023 multi-stakeholder workshop on the amendments to the Hazardous Products Regulations; details on how to obtain a copy of the document can be found in the June newsletter.
Feel free to reach out to Nexreg Compliance with any global regulatory questions. SPRAY