Written on: December 1, 2017 by W. Stephen Tait
Hello, everyone. In the September edition of Corrosion Corner, I began a five-part series on the corrosion behavior of the various metals used to fabricate aerosol containers and valves. This month I’ll discuss the corrosion behavior of stainless steel, which is an iron alloy containing chromium and nickel; the springs inside aerosol valves are typically made from stainless steel.
Aerosol container valves
Figure 1 shows a cross-section of an aerosol valve assembly. The stainless steel spring holds the valve stem against the valve cup so that the stem gasket seals the valve orifice. Depressing the spring exposes the valve orifice, allowing the product to spray. The stainless steel spring is submerged in product after the first spray, providing continuous opportunities for valve spring corrosion.
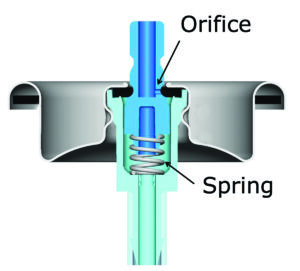
Figure 1. Aerosol valve cross section (provided by Precision Valve)
The corrosion resistance of the stainless steel valve springs is determined by the chemical composition of a formula, much like the formula chemistry determines the corrosion behavior of the other metals and polymer components inside a spray package. However, the stainless steel springs are often more resistant to corrosion than the other package metals. Let’s discuss why.
Stainless steel corrosion chemistry
There are over 300 different types of stainless steel alloys. Type 302 stainless steel is the most common type of valve spring stainless steel. This alloy has from 17% to 19% chromium, as well as 8% to 10% nickel plus an additional 3.2% maximum combined concentration of several other trace elements. The iron concentration in stainless steel is from 68% to 72%, which is considerably less than the 99-plus percent iron concentration in tinplate steel and tin-free-steel aerosol containers and valves. The aluminum used for aerosol containers and valves does not contain chromium and nickel.

Figure 2. The corrosion rate of the iron-chromium alloys as a function of chromium concentration.
Corrosion Basics: Iron-chromium alloy (stainless steel) corrosion behavior
Figure 2 illustrates how iron-chromium alloy (stainless steel) corrosion rates are affected by the alloy chromium concentration. The Y-axis in Figure 2 contains the corrosion rate for the corresponding chromium concentrations on the X-axis. Notice that the corrosion rate decreases from 0.4 to 0.5mL per year at 1% chromium to a negligible rate when the chromium concentration is approximately 10% (~ 0mL per year).
Chromium forms a very thin, continuous chromium oxide surface layer on the stainless steel when the chromium concentration exceeds 10%. The nickel in stainless steel also forms an oxide that mixes with the chromium oxide on the surface. This mixed oxide layer is very thin, ranging from 20 Angstroms to 100 Angstroms thick (one Angstrom = 1×10-8 centimeters), and is a corrosion protection barrier for a wide range of aerosol formula chemical compositions.
Formation of a protective oxide film on stainless steel is referred to as passivation.
Consequently, the chromium and nickel in a stainless steel makes valve springs resistant to corrosion by a very wide range of aerosol formulas. Please note that I use the word resistant and not corrosion-proof.
Corrosion basics: Chlorides and stainless steel corrosion
Chloride ions in your formula breakdown the protective chromium-nickel oxide layer on stainless steel springs. Film breakdown is referred to as depassivation. In a wide range of instances, the protective layer is repaired faster than it is depassivated.
However, there are chemical compositions that depassivate the protective layer faster than it is repaired. Formula pH, the concentration of chloride ions in your formula and the particular type of valve spring stainless steel are a few of the factors that determine if the oxide depassivation rate will exceed the repair rate.
Pitting corrosion results from depassivation that exceeds repair and pitting corrosion often causes spring cracking with subsequent breakage. A broken spring could cause the container to stop spraying or product leaking through an incompletely sealed valve orifice.
There are no rules for what combinations of these factors cause pitting corrosion or what concentration of chloride ions cause corrosion. Consequently, corrosion testing or a large valve spring corrosion database are the only ways to determine if a particular formula will cause aerosol valve springs to pit and possibly crack.
The aerosol container chloride myth
Stainless steel pitting corrosion by chloride ions gave rise to the spray package corrosion myth that chloride ions cause aluminum and steel aerosol container corrosion. Chromium and nickel are not in aluminum packaging metals and the amount of chromium and nickel in both tinplate and tin-free-steel is too low to passivate tinplate and tin-free-steel.
Thus, aluminum and steel aerosol containers do not have the passive oxide film that is susceptible to chloride depassivation. Consequently, chlorides do not typically cause aluminum or steel aerosol spray package corrosion.
In January, we’ll complete the series with a discussion of laminated metal packaging.
Pair O Docs has a state-of-the art electrochemical corrosion testing laboratory; please contact me if you would like to know more about our faster and predictive corrosion testing. You can also visit our new website which has a short Vision Video that discusses all our corrosion prevention and control services. Please also contact me if you would like to a have our Elements of Spray Package Corrosion short course taught at your R&D facility. Back issues of Corrosion Corner are available on CD-Rom. Thanks for reading and I’ll see you in 2018. SPRAY