Written on: September 1, 2021 by W. Stephen Tait
Hello, everyone. This month we continue a series that began in June concerning spray packaging material defects and their relationship with corrosion. Corrosion Corner in June and July covered defects in polymer and tinplate coatings, laminated film bags and the metals used for traditional aluminum and steel aerosol containers. Most of these material defects are very small, but can still be seen with the unaided eye. A microscope is needed, of course, to see the details of these small defects.
There are also material defects that can only be seen with a scanning electron microscope or a light microscope. However, these defects can also contribute to or cause spray package corrosion. Thus, microscopic material defects could also contribute to or cause spray package failure (i.e., package leaking).
The metals used for spray packaging are not pure metals. Instead, they are alloys that are mixtures of metals and non-metals, such as carbon and oxygen.
Metal/non-metal compounds, such as metal oxides or metal carbides, fall out of solution and form microscopic particles in the bulk alloy while the molten alloy cools. These particles are dispersed throughout the alloy bulk and are referred to as inclusions. Inclusions can be flattened and elongated during the process of rolling and thinning the metal into the sheets for spray package fabrication, as well as when the metal is extruded into a container.
Figure 1 provides a photomicrograph with examples of inclusions. They are the small dark spots and the thin lines in the metal. The thin lines are also referred to as stringers. The arrows show the locations for only a few of the many, many defects in Figure 1.
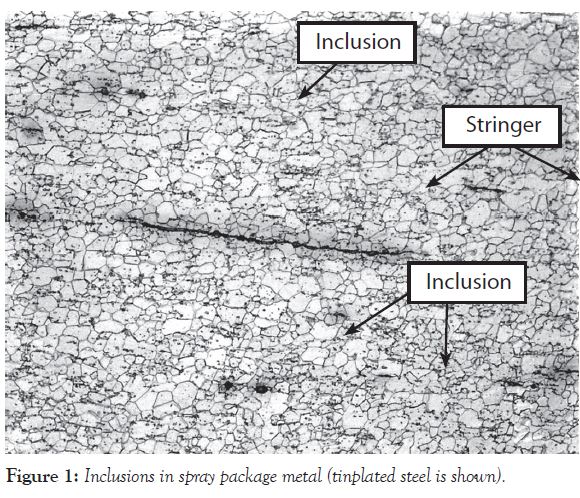
Inclusions are sites for pitting corrosion and stress cracking. Stress cracking is rare in spray packaging, and the chemical composition of a formula determines whether or not pitting corrosion occurs at inclusions.
Please keep in mind that inclusions are not impurities. Inclusions are material defects that result from alloying a metal with different elements to obtain desirable physical properties, such as strength and formability.
Metals form regular arrangements of atoms, and these atomic arrangements form bulk formations referred to as crystal planes. Crystal planes are often not perfect, and in some instances consist of plane-fragments imbedded inside complete planes. The plane- fragments are referred to as dislocations.
Figure 2 provides an example of dislocations and how they pile up when a metal is rolled into a sheet. Steel and aluminum metals and alloys all have dislocations. Figure 2 also shows that dislocations could be sites for the initiation of pitting corrosion. An etching solution of ethyl alcohol and nitric acid caused the pitting corrosion in Figure 2.
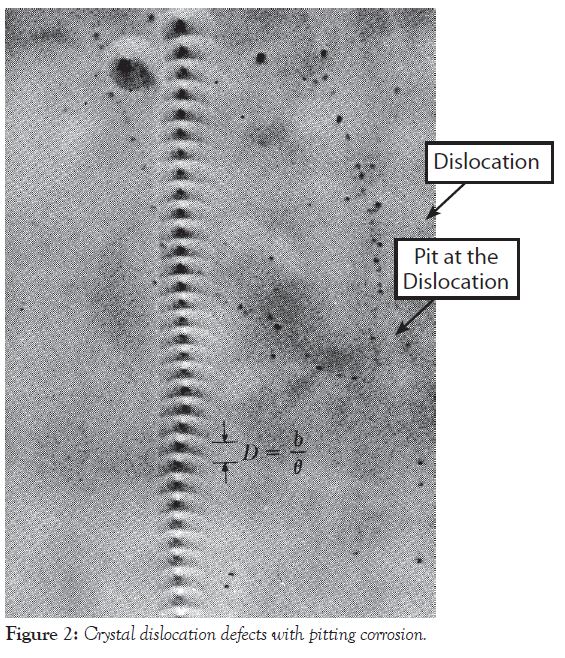
It has been estimated that there are approximately one million dislocations per square centimeter of metal surface. The chemical composition of a formula determines whether or not pitting corrosion occurs at dislocations.
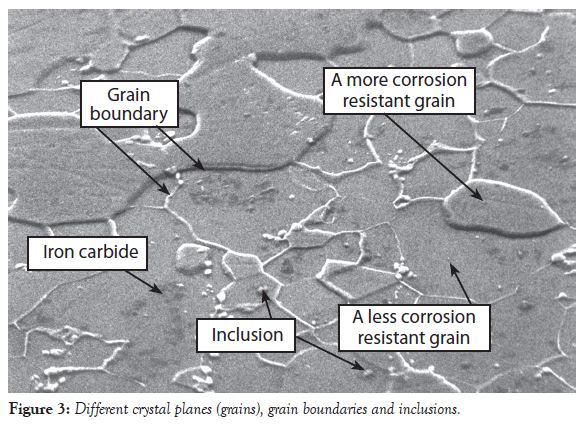
Figure 3 provides a scanning electron micrograph of tinplated steel. The tin coating was removed from the steel by mechanical polishing. The steel was further polished after the tin was removed and then etched with the alcohol/nitric acid solution previously mentioned.
There are a several different types of defects shown in Figure 3:
• Different crystal planes form structures referred to as grains, and different types of grains corrode more rapidly than others (as shown in Figure 3);
• The chemical composition of the boundary between two different types of grains is typically different from the chemical composition of the grains;
• The boundary between different grains is not always metallic—the boundary could be non-metallic compounds or a mixture of metallic and non-metallic compounds;
• Inclusions (the white particles inside grains) are also seen in Figure 3;
• Iron carbide compounds are also present in the grains (agglomerates of darker areas and spots)
The material defects shown in Figure 3 can contribute to or cause spray package corrosion. Aluminum and steel both have one or more of the material defect types shown in Figure 3.
The chemical composition of a formula determines whether or not the material defects in Figure 3 contribute to or cause spray package metal corrosion.
The current state of corrosion science is currently not advanced enough to predict when corrosion will occur. Hence, corrosion testing is essential to reduce the risk of costly surprise spray package corrosion.
See you in October. Contact me at 608-831-2076, rustdr@pairodocspro.com, pairodocspro.com or aristartec.com. SPRAY