Written on: March 1, 2022 by W. Stephen Tait
Hello, everyone. Spray packages allow consumers to easily dispense a controlled amount of product to a specific area. Corrosion is an issue for all types of spray packaging and packaging materials. However, corrosion can be controlled and/or prevented with a comprehensive corrosion prevention and control program.
Let’s start with a brief overview of why spray package corrosion occurs and then discuss the elements in a comprehensive corrosion control and prevention program. Three elements are needed for spray package corrosion to occur:
1. A material susceptible to corrosion
2. An environment containing corrosive chemicals
3. A surface accessible to the corrosive chemicals
Materials susceptible to corrosion
Corrosion is the degradation of materials, and all materials are susceptible to some form of corrosion. In other words, there’s no such thing as a corrosion-proof material for all formulas.
Corrosion often causes materials to lose important properties, such as polymer coating barrier properties. Corrosion can also lead to package failures, such as leaking product or propellant—or the package still has product in it but no longer sprays.
Corrosive chemical environment
The environment inside spray packages is your formula. There are so many available chemicals to formulate consumer package goods that a comprehensive list of ingredients that could either contribute to or cause corrosion is unavailable.
However, water is a common formula ingredient or contaminant that is corrosive toward all spray package materials. Water molecules readily absorb into and diffuse through polymers, subsequently causing polymer corrosion, such as blistering. Water is also electrochemically-active and removes electrons from uncoated metals and metals under coatings and laminated films.
Surfaces accessible to corrosive chemicals
All spray packages have internal surfaces exposed to formulas. Internal surface molecules are thermodynamically unstable, and thus susceptible to corrosion. Corrosive formula ingredients:
1. Adsorb on surfaces
2. Absorb into polymers
3. Diffuse through polymers; and
4. Cause corrosion by removing electrons from both uncoated and coated metal atoms
Material corrosion initiates and propagates after one or more of these above steps.
A comprehensive corrosion control and prevention program has six elements as shown in Figure 1. Our Elements of Spray Package Corrosion short course provides a detailed discussion of this Figure, so only an overview will be provided here, starting at the top of Figure 1 and proceeding clockwise around it.
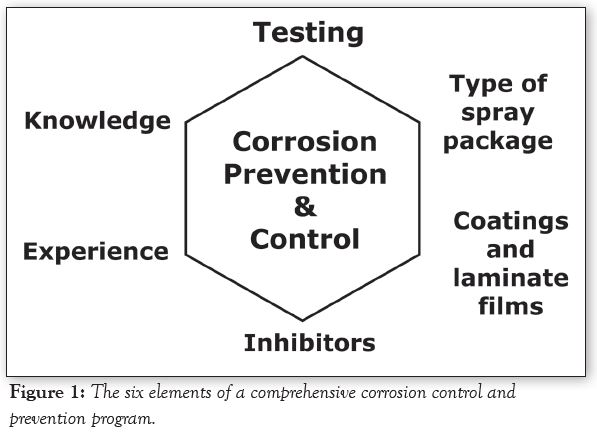
Corrosion Testing
The current state-of-the-art for corrosion science is extensive, but not enough to predict if corrosion will or will not occur using data tables, equations or chemistry first principles. Consequently, corrosion testing is an essential element in a corrosion control and prevention program. A corrosion test could be either a storage stability test or an electrochemical corrosion test. A storage stability test has an approximately 93% correlation between its results and actual commercial spray package corrosion when the test is conducted for at least one year. Lower test times reduce the correlation and thus increase the risk of surprise corrosion with commercial units. In addition, corrosion rates are not accelerated by exposing packages to higher temperatures. In other words, results from higher temperatures cannot be used to predict package service lifetimes.
Electrochemical corrosion tests are completed in fewer than 100 days with a correlation that exceeds 99% when the appropriate protocols and test cells are used. In other words, electrochemical tests are completed in less time than a storage stability test with less risk. Electrochemical tests can be applied to all types of spray packages and spray package materials.
Types of spray packages
There is a variety of spray package types available for spray formulas. Sometimes one type of package is significantly more corrosion-resistant than others. Corrosion testing is used to:
• Determine if new and derivative formulas are corrosive toward specific package types
• Select the most corrosion-resistant form of spray packaging; and
• Determine if alternate packages are suitable for current formulas
Internal coatings & laminate films
Uncoated metals are the least expensive form of spray package materials. However, in some instances, an internal coating either prevents package corrosion or extends the service life of a metal package to an acceptable level. Corrosion testing is also used to select the most corrosion-resistant coating or laminate film for a given formula.
Corrosion inhibitors
Corrosive formulas do not have to be abandoned. Corrosion inhibitors are an effective way to control or prevent spray package corrosion. However, inhibitor development typically extends a development timetable. Corrosion testing is used to select the most effective corrosion inhibitor and determine its effective concentration range. Inhibited formulas are often patentable and thus provide a market advantage.
Experience & knowledge
Knowledge without practical experience or experience without theoretical frameworks are incomplete and will not result in useful, predictive, professional practice. In other words, experience and knowledge are intricately linked.
Public domain consumer packaged product corrosion databases are unavailable because of market competition. Consequently, proprietary corrosion databases are typically developed by individual companies for internal use only. A proprietary database:
• Allows faster-to-market introduction of new and derivative products
• Provides formulation guidelines to avoid developing corrosive formulas
• Reduces corrosion risk during new and derivative product development; and
• Reduces the corrosion risk
Thanks for your interest and I’ll see you in April. Contact me at 608-831-2076, rustdr@pairodocspro.com or from our two websites: pairodocspro.com and aristartec.com. SPRAY