Written on: June 1, 2022 by W. Stephen Tait
Hello, everyone. It’s commonly believed that coatings and laminate films are barriers between a spray package metal or metal foil and a formula. It’s also commonly believed that a barrier is needed to prevent metal corrosion. However, these beliefs are not always true!
Why don’t coatings always prevent corrosion of the coating and the substrate metal? Coatings bond to metal surfaces that are a complex mixture of:
• A variety of metal atoms and non-metal atoms
• Groups of metal crystals referred to as grains
• Thin, non-metallic boundaries between the different grains
• Metal alloying components that precipitate in grain boundaries and inside grains
• Atomic structural defects within grains, such as missing metal atoms and lines of atoms
Complete metal surface coverage by a coating requires that it bonds to all materials on the surface. However, complete bonding rarely occurs, creating coating defects.
This column will discuss the most common defects in coatings—holes, solvent pops, pores, voids and pore-void combinations. For simplicity, I’m also going to collectively refer to coatings and laminate films as coatings.
Figure 1 provides a photomicrograph of a hole through a coating that leaves the substrate metal completely exposed.

It’s tempting to conclude that the hole in Figure 1 will cause pitting corrosion. However, coating-holes are very common. I’ve observed numerous instances where no pitting occurs in the holes; alternately, I’ve seen pitting corrosion occur where there are no holes. Indeed, pitting corrosion typically only occurs in a hole when there is also a large area of corroding coating surrounding the pit.
Figure 2 provides an example of a coating solvent pop. Notice that the solvent pop is approximately five times larger than the hole in Figure 1.

Solvent pops are common. In one instance approximately 25% of the containers from a single manufacturing lot had at least one solvent pop. Other times, solvent pops are a rare event in a given manufacturing lot.
The thin cap over a solvent pop is usually quickly removed after a package is filled. However, metal pitting corrosion or coating delamination in a solvent pop needs to be supported by a large surrounding area of corroding coating.
Pores and voids are microscopic and difficult to observe. Indeed, coating voids are typically detected indirectly with analytical methods, such as positron annihilation.
The diagram in Figure 3 illustrates the possible pores, voids and pore-void combinations in coatings. The blue lines in Figure 3 depict pores; the blue ovals and the irregular blue shape depict voids.
Liquid moving into and through pores creates microscopic rivers of liquid that could lead to extensive coating corrosion, loss of coating properties and corrosion of the metal substrate. The chemical composition of the microscopic rivers is typically significantly different from the composition of a bulk formula.
Three of the lines and voids in Figure 3 are labeled:
1. This depicts a pore that eventually ends as a coating void. Liquid fills the void by capillary action and the chemical composition of the liquid is typically significantly different from a bulk formula’s composition.
2. This illustrates how pores can meander throughout the coating without ending at a void. Both examples #1 and #2 will cause coating corrosion with subsequent loss of barrier properties.
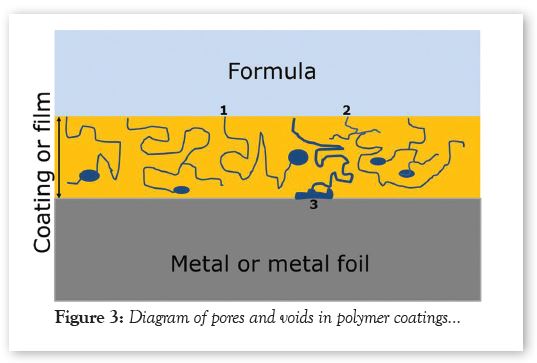
3. This illustrates how a pore could provide continuous contact between a formula and the metal under the coating, with liquid accumulating at the metal-coating interface. Metal corrosion and coating delamination, such as blisters, could occur when the liquid moving through the coating is corrosive toward the metal; sufficient liquid accumulates at the metal-coating interface; or the volume of liquid continuously moving through the rivers is large enough to sustain metal corrosion.
I’ve seen many instances where coating and metal corrosion began and subsequently stopped because oxides/hydroxides from metal corrosion plugged the rivers and/or the coating pores. I’ve also seen instances where coating-swelling by water absorption block the rivers and stopped the flow of liquid needed to sustain metal corrosion under the coating.
The chemical composition of a formula, type of package metal and type of coating all interact to determine:
• How much liquid absorbs into the coating through pores
• If the coating will corrode and lose its barrier properties and
• If the substrate metal will corrode.
Thanks for your interest and I’ll see you in July. Contact me at 608-831-2076, rustdr@pairodocspro.com or from our two websites: pairodocspro.com and aristartec.com. SPRAY