Written on: November 1, 2022 by W. Stephen Tait
Hello, everyone. I started a two-part series about the science for using higher temperatures to accelerate the rate of spray package corrosion as a possible way to reduce storage test time in the last issue. The first part centered on the science for the Arrhenius equation, often used to justify using higher temperatures to accelerate corrosion. It was demonstrated that the Arrhenius equation is usually invalid for spray package materials; hence higher temperature does not accelerate metal and polymer corrosion rates.
I’ll pick up where we left off with a continuation of how higher temperatures actually affect polymer and metal corrosion.
3. Do higher temperatures actually accelerate corrosion? (Continued from last issue)
Sometimes higher temperatures appear to produce more corrosion than lower temperatures, leading to the impression that a higher temperature is accelerating the corrosion. Polymer-coated metals provide a good example of why this is not the case.
Figure 1 summarizes data from corrosion measurements on coated tinplate at temperatures from 20°C to 80°C (68°F to 176°F). Notice in Figure 1 that instead of the corrosion rate increasing linearly with increasing temperature, the corrosion rate actually decreases between 20°C (68°F—room temperature) and 60°C (140°F). Thus, increasing temperature in this range does not accelerate coated tinplate corrosion. Notice also that the corrosion rate doubles as the temperature is increased from 60°C to 80°C (140°F to 176°F). Figure 1 demonstrates that the Arrhenius equation is not valid for coated metal corrosion.
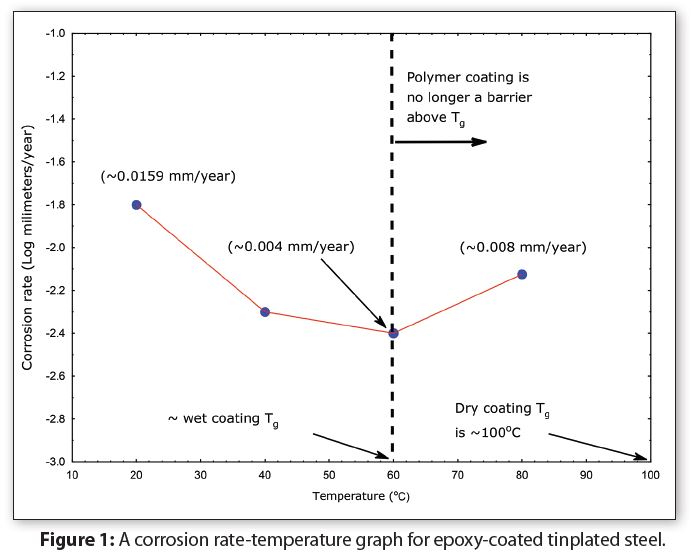
The reason why the Arrhenius equation does not predict corrosion for coated metals is because polymer corrosion is a complex interaction between absorption of water, molecules and ions into and through the polymer, plus breaking metal-polymer bonds. In other words, polymer-corrosion is not a first order reaction.
Absorption also changes a polymer’s physical properties—such as lowering the glass transition temperature (Tg) and often breaking metal-coating bonds.
Notice in Figure 1 that the wet-epoxy Tg is approximately 40°C (104°F) lower than the dry-epoxy Tg. The wet coating corrosion rate increases as temperature is increased because the wet-epoxy is no longer a protective-barrier between the formula and the container metal. Both polymer and metal corrosion occurred in this situation. In other words, exceeding the coating glass transition temperature causes coated tinplate corrosion. Consequently, Figure 1 also helps to explain why coated metals sometimes appear to have significantly more corrosion at higher storage temperatures.
To summarize, polymers lose their properties—including corrosion barrier-protection—when the temperature is at and above their Tg. Thus, higher storage test temperatures subject package polymer materials (coatings) to temperatures above the wet-polymer Tg, with the result that the polymers no longer have their original corrosion-barrier protection property. In this situation, the corrosion at a higher temperature is not indicative of actual corrosion.
Both metal corrosion and polymer coating corrosion are not pure chemical reactions and both types of corrosion rarely meet the two requirements for valid use of the Arrhenius equation. Consequently, higher temperatures rarely accelerate the corrosion of the metals, internal coatings and laminated films used for spray packaging.
4. Should storage tests be conducted −and for how long?
Higher temperatures should be part of a storage test. It might not seem likely and I’m not contradicting myself. Higher temperature corrosion data provides a way to determine if the formula is temperature-sensitive, but not as a way to accelerate corrosion.
Products may be exposed to higher temperatures while being transported or stored during the Summer months. However, actual exposure times at higher temperatures are often short, because peak daily temperatures typically only last for a few hours every 24 hours. Thus, the majority of filled spray packages spend most of their service lifetime at or near room temperature (assuming no long exposures to direct sunlight).
Higher temperatures could degrade components of a formula, making it corrosive. Higher temperatures could also destabilize the physical properties of a formula. For example, higher temperatures could break water-in-oil emulsions, producing a corrosive, free-water phase. Consequently, higher temperature storage testing is recommended to determine if:
• Your formula is stable at higher temperatures
• Formula instability at higher temperature produces conditions that cause spray package corrosion
5. How long should test samples be exposed to higher temperatures?
The amount of time that packages are stored at higher temperature depends on the local climate where your products are purchased and used.
Figure 2 shows a graph of the hourly temperatures for Middleton, WI for 24 hours during Summer (Source: National Oceanic & Atmospheric Administration). Indoor temperatures are typically 5–10 degrees lower than the outside temperatures, so Figure 2 includes a simulated graph for indoor temperatures.
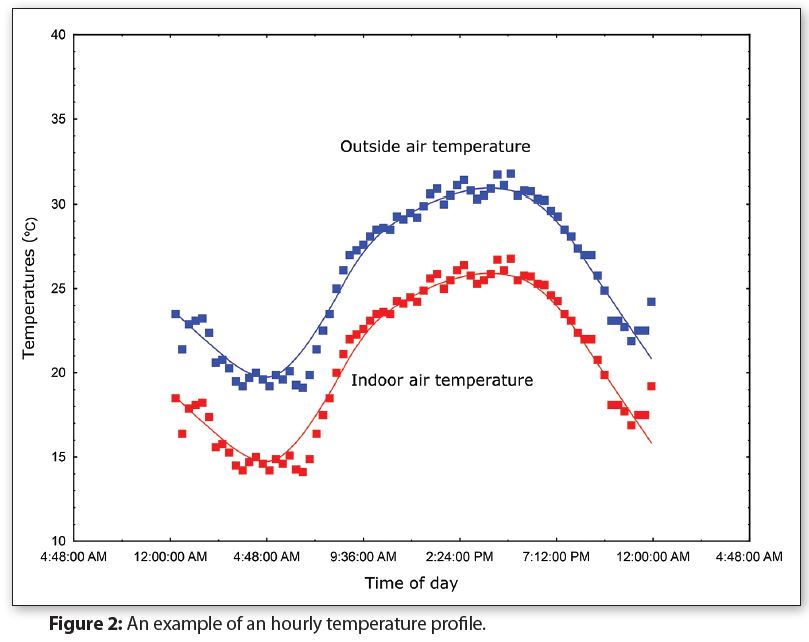
Notice in Figure 2 that the outside air temperature is only at or above 30°C (86°F) for approximately seven hours during this particular day. If there are 40 days each year where the temperature is at or above 30°C, then there are cumulatively about 10 days per year when the temperature is at or above 30°C.
In other words, 10 days of continuous storage at or above 30°C (86°F) simulates approximately one year of exposure. Storing packages at 30°C for 30 days would simulate three years and continuously storing packages at 30°C for one year would simulate 36 years.
Please keep in mind that these times and temperatures do not simulate one, three and 36 years of package corrosion.
Higher temperature testing is needed, particularly when products are marketed in regions having high temperatures. Consequently, I recommend the following guidelines for higher temperature storage testing:
• Don’t use a higher storage temperature to accelerate package material corrosion rates in order to reduce test times.
• Use higher temperature storage to determine if there are possible formula thermal instability issues that could cause package material corrosion.
• Conduct tests at higher temperatures that reflect the actual high temperatures for the regions where your products are marketed.
• Use test lengths that reflect the actual number of hours per year when your products are exposed to higher temperatures.
Summary & conclusions…
There is no reliable way to accelerate spray package corrosion rates. However, electrochemical corrosion testing can provide accelerated results because sensitive electronic instruments detect corrosion much sooner than it can be observed with either the unaided eye or a microscope. In some instances, a comprehensive company knowledge database also allows reduction of the number of variables in a corrosion test without increasing the risk of unexpected package failure. Higher temperatures for storage tests should be used to determine product stability at higher temperatures but not to accelerate package material corrosion.
Thanks for your interest and I’ll see you in December. Contact me at 608-831-2076, rustdr@pairodocspro.com or from our two websites: pairodocspro.com and aristartec.com. SPRAY