Written on: December 1, 2022 by W. Stephen Tait
Hello, everyone. I have often been asked if corrosion could be mathematically modeled so that long-term corrosion testing could be either shortened or skipped. The short answers are Yes-and-No.
There are many potential formula and package material factors that cause or contribute to the corrosion of spray package materials.
The seven major categories of corrosivity factors are:
1. Electrochemically active (ECA) formula ingredients are often those whose molecules are unsaturated and thus can contribute to or cause corrosion of package metal components. Ions such as hydrogen ions and molecules such as water are also ECA formula ingredients, as are many insecticides.
2. Liquid Water is an EAC molecule. Water can be either part of the formula or present as a contaminant.
• Only 90 water molecules are needed to form liquid water and thus initiate corrosion
• Water opens up polymer coatings and laminate films by forming microscopic rivers through the coatings and the films
3. Formula pH is the hydrogen ion concentration in your formula-water or contaminant-water. pH is typically expressed as the negative log of the hydrogen ion concentration. For example, water with a pH of seven has a 10-7 moles/liter hydrogen ion concentration.
Different metals have different ranges where they are either more—or less—corrosive by formula pH. For example, steel alloys usually corrode rapidly at low pH, but slowly at high pH. Aluminum does not corrode in pure water when the pH is from around 3–7, but corrodes when the pH is lower than three and higher than seven in pure water.
4. Fragrances have been shown to function as corrosion inhibitors in many instances—and a few types of fragrances contribute to or cause spray package corrosion. The concentration of the fragrance often determines either the level of corrosion inhibition or the corrosivity of a given fragrance.
5. Surfactants make it easier for ECA ions and molecules to contribute to or cause corrosion by enhancing water adsorption to metal surfaces and the absorption that creates water molecular-rivers through polymer coatings and films.
6. Propellants in some instances might contain EAC molecules. There are multiple types of propellants:
• Compressed gases such as carbon dioxide (EAC is carbonic acid), air (EAC is oxygen) and nitrogen
• LPG propellants that are blends of butane isomers, pentane isomers and propane (no EAC molecules)
• Hydrofluorocarbons, such as HFC152a and Solar (typically no EAC molecules unless contaminated with water)
• Dimethyl ether (DME)—this is a good solvent for a wide range of polymers and can, in some instances, contribute to metal corrosion by degrading internal package coatings and films
7. Spray package materials can be metals, polymers or a combination of both.
The metals for spray packages can be:
• A thin, chromium-coated steel (tin-free-steel or TFs)
• Tin coated steel (tinplate)
• Aluminum
The polymer coatings for spray package metals can be:
• Epoxy coatings
• PVC particles suspended in a epoxy matrix
• PAM coatings
• Laminate films composed of a layer of nylon (e.g., OPA) on top of polypropylene
• Vinyl coatings
The chemical composition of your formula and the type of package materials determine if a formula will contribute to or cause spray package materials corrosion.
In summary, spray package corrosion is a complex interaction of multiple, potential corrosion-causing factors in at least seven categories. Consequently, the mathematical equations to predict spray package corrosion must have parameters for all of these factors.
Three corrosion questions should be answered before marketing a new spray product or a derivative spray product:
1) Is corrosion expected?
2) How fast is corrosion expected to degrade and/or penetrate package materials?
3) What type or types of corrosion are expected?
Question #2 is about package service lifetime. Service lifetime is defined as the filled package age when:
a) The package leaks product or propellant;
b) Valves leak propellant;
c) Partially full packages cease to spray; or
d) Package corrosion degrades product performance and/or efficacy.
In other words, service lifetime is the length of time during which spray packages and their products function properly.
Empirical equations for the first two corrosion questions need to incorporate all the formula and package. Let’s discuss the equations for Questions #1 and #2. Discussion on the equation for the third corrosion-question is deferred for a later edition of Corrosion Corner.
1. Is corrosion expected?
The Gibbs-free-energy equation can be used to estimate the probability of package corrosion. An empirical version of the Gibbs-free-energy equation for the probability of spray package corrosion is:

The various symbols in the Gibbs-equation mean or indicate:
• ∆G = Gibbs free energy
• n = 2 or 3 for steel /n = 3 for aluminum
• F is the Faraday’s constant
• K is a proportionality constant
• Ψ is probability
• Single, lower-case, superscripted letters are exponents for the equations inside parentheses. These can range from 0–infinity
• G indicates that all the factors between square-brackets are multiplied
• A combination of superscripted lower-case letters with sub-scripted lower-case letters represents unknown equations. Some of these unknown equations are exponents for equations in both parentheses and square-brackets
• Single, lower-case letters represent numbers ranging from 1–infinity
There are approximately 15 parameters in the Gibbs-equation. Consequently, there are 15 [!] (15-factoral) possible combinations. In other words, there are 1,307,674,368,000 possible combinations of the corrosivity-factors that determine if a formula will contribute to or cause spray package corrosion.
A negative ∆G indicates that package corrosion is expected by a formula. However, the Gibbs-equation does not tell us how fast the corrosion by a formula degrades and/or penetrates a package, thus reducing the package service lifetime. Hence, let’s move on to discuss the empirical equation for how fast corrosion is expected to degrade and/or penetrate package materials.
2. How fast is corrosion expected to degrade and/or penetrate package materials?
The empirical equation to estimate the most probable corrosion rate can be seen in Figure 1.
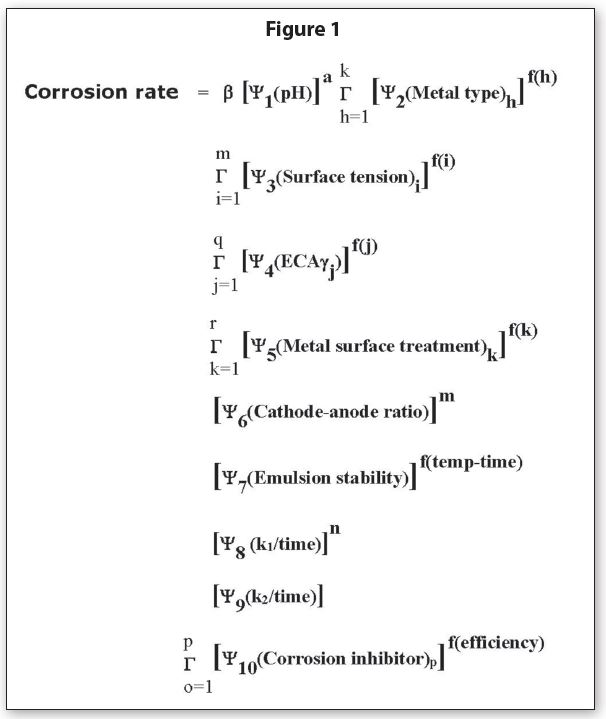
The new symbol, b, is a conversion factor that converts calculations from the Equation into a corrosion rate. The probability equation for package material corrosion rates has approximately 25 parameters in it. In other words, there are approximately 15,511,210,043,330,985,984,000,000 possible combinations for the corrosivity factors needed to estimate a package corrosion rate with a specific formula.
Clearly, the two empirical equations are very complex and require knowledge of many, many corrosion factors and equation parameters before either can be solved for a specific package-formula system. To further complicate matters, no-to-very-little research is available for the specific corrosion factors and equation parameters in both equations. Hence, the corrosion factors for consumer packaged goods spray products are virtually unknown at this time.
Consequently—while theoretically possible—substituting mathematical models for corrosion tests is not practical at this time. Thus, corrosion testing is currently the only practical and reliable way to determine:
1) Will a formula or line extension corrode the chosen spray package materials?
2) How fast will corrosion proceed through the materials—in other words, what is the package service lifetime with a specific formula?
3) What type or types of corrosion are expected?
Thanks for your interest and I’ll see you next year. Contact me at 608-831-2076; rustdr@pairodocspro.com or from our two websites: pairodocspro.com and aristartec.com. SPRAY