Written on: February 1, 2023 by W. Stephen Tait
Hello, everyone. Defect-free spray package materials do not exist and these defects are often misidentified as corrosion.
Even though defects don’t often contribute to or cause spray package corrosion, some formulas will interact with defects to produce package corrosion. Thus, there are always potential concerns as to whether or not defects will contribute to or cause spray package corrosion and if this corrosion will cause spray packages to fail (leak).
This issue begins a two-part discussion on material defects and their relationship to package corrosion, beginning with traditional aluminum aerosol containers. Discussion on material defects in laminated foils and tinplated steel aerosol containers will follow in the next issue.
Photographs of material defects are used to discuss the relationships between defects and corrosion.
Aluminum aerosol container material defects
Figures 1–6 provide examples of material defects in coated aluminum aerosol containers. Figure 1 shows an example of an inclusion in the container metal.
Inclusions are microscopic materials that are insoluble in the aluminum metal. The two major types of inclusions include:
1. Particles of the metals and non-metals added to aluminum to give it the desired strength and formability; and
2. Crystals of aluminum complexed with other metallic and non-metallic elements, for example, aluminum silicate.
Inclusions are often spherical and become distorted and flattened when the aluminum alloy is formed into a container.

I’ve only observed one rare instance when an inclusion such as the one in Figure 1 caused container pitting corrosion with subsequent leaking.
Aluminum tubes and aerosol containers are formed by extruding a small aluminum disc into the desired container shape. Small pieces of metal (divots) are often ripped-from the aluminum during extrusion, an example of which is shown in Figure 2.

Notice in Figure 2 that the internal package coating backfilled the divot. I have only observed rare instances where this type of defect contributes to or causes package corrosion.
Aluminum aerosol containers are open at the top and closed at the bottom. The tubes are cleaned with a spray nozzle to remove the lubrication applied during extrusion and the internal coating is subsequently sprayed inside the tube prior to forming the container top dome. The nozzle moves from the bottom of the tube to the top during the coating application.
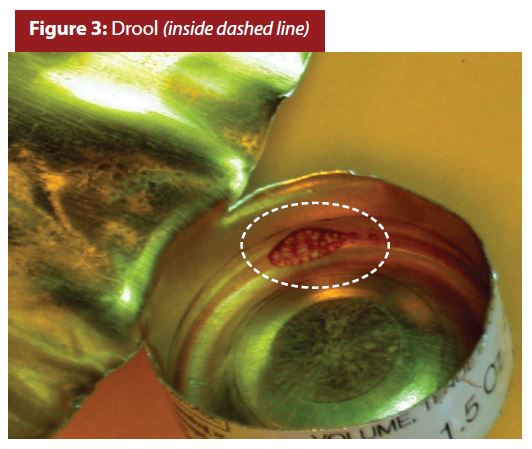
In some instances, coating drips from the nozzle when spraying is stopped near the top of the tube. Figure 3 shows an example of a coating drip in an aluminum aerosol container, commonly referred to as a drool. I’ve not observed an instance where a coating-drool contributed to or caused aerosol container corrosion.
Entrained air in the bulk coating material sometimes causes a coating nozzle to eject a small amount of excess coating. The excess coating that sticks to the surface is referred to as a spit, examples of which are shown in Figure 4. Notice that the spits are at two different locations inside the two containers.
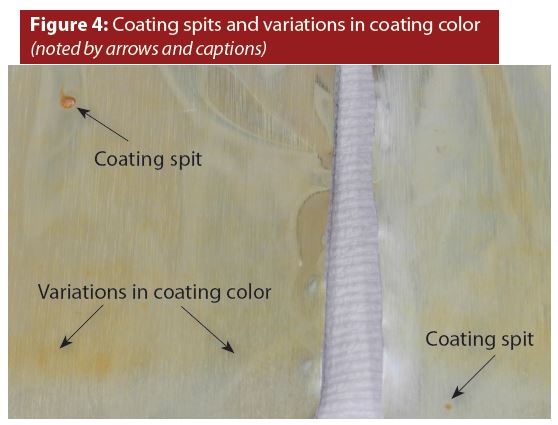
Spits are very common in aluminum aerosol containers. However, I have not observed an instance where a spit contributed to or caused container corrosion.
Figure 4 also shows variations in coating color. Coating color variations could be caused by variations in the thickness of the coating—a well-known phenomenon in the coatings industry. I have observed a few rare instances where variations in color appeared to cause random container failures (leaking).
Aerosol container coatings are cured with high temperatures. Coatings are dissolved in solvents that evaporate during the curing process and small bubbles can form during evaporation. Sometimes these bubbles harden, producing solvent pops like the one in Figure 5.
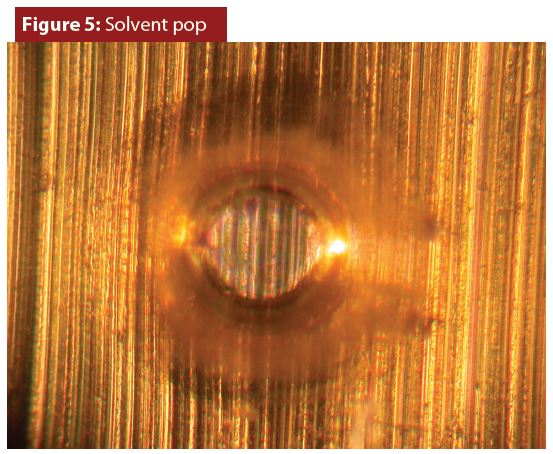
Solvent pops rarely contribute to or cause corrosion. Pitting corrosion inside solvent pops can only occur when there is also extensive coating corrosion in a large area surrounding a solvent pop.
Small holes form in coatings wherever the coating does not wet (cover) the package metal. Figure 6 shows an example of a hole in a coating. The hole is inside the meandering dashed line in Figure 6. Holes like that shown in Figure 6 are very common and often expose the container metal to your formula.

This type of defect causes pitting corrosion when there is also extensive coating corrosion in a large area around the hole.
One or several of the defects shown in Figures 1–6 are present in most aluminum aerosol containers and tubes. Consequently, corrosion testing with your specific formulas is needed to determine when material defects will contribute to or cause container corrosion that leads to package failure (leaking).
In the next issue, I will complete this discussion on material defects for laminated foil packages, as well as for both coated and uncoated tinplated steel aerosol containers.
Thanks for your interest and I’ll see you in March. Contact me at 608-831-2076; rustdr@pairodocspro.com or from our two websites: pairodocspro.com and aristartec.com. SPRAY