Spray package corrosion barriers-Part 2
Written on: November 1, 2023 by W. Stephen Tait
Hello, everyone. In the last issue, we began a three-part series (Part 1) with a discussion on how the complex composition of metal surfaces causes coating pores and voids and how they allow formula ingredients to absorb into and through coatings.
Epoxy, Micoflex, polyethylene terephthalate (PET) and bi-layer nylon-polypropylene laminate films are typically used as internal coatings for spray packages. I will continue to refer to both coatings and laminate films as “coatings” because the corrosion science is the same for both.
The intention is for the coating to act as a barrier between a formula and the underlying metal or metal foil. Consequently, it often comes as a surprise when a c oating is corroded by a formula.
Internal coatings on metal are dry when packages are removed from their pallets for product filling. Liquid dispersed throughout a coating transforms it from a dry coating to a wet coating, and a dry coating has significantly different properties than those of a wet coating, such as the ability to be a barrier between the package substrate metal and a formula.
A “skin over metals”
Think of coating as a skin over metals. Human skin is a natural polymer that provides a good analogy for the corrosion of coatings used for spray package coatings and laminate films.
Formula ingredients, such as water and emollients, can absorb into skin and subsequently modify its properties, such as causing it to wrinkle or make it feel soft, respectively. Skin properties could also be modified by formula pH. For example, a liquid will cause a burning sensation on skin when the liquid pH is higher or lower than around 5.5 and the burning sensation typically intensifies as the pH moves further from 5.5.
Like skin, the properties of coatings are also modified when materials absorb into a coating. The intensity of the modification depends on the type of coating, its morphology, how it was deposited inside the package, the chemical composition of the formula and the amount of liquid dispersed throughout the coating.
Diffusion of a liquid-permeate into and through a coating could cause:
1. The coating to become a semi-permeable membrane that allows an either a non-corrosive or corrosive liquid to diffuse into and through the coating
2. Loss of the coating’s barrier property
3. Lowering of the coating’s glass transition temperature (Tg)
As mentioned in the above list, formula ingredients absorbing into coatings could cause the coatings to become a semi-permeable membrane instead of a barrier layer. A semi-permeable membrane—originally a dry coating—allows select formula ingredients to diffuse into and through the coating. Hence, the chemical composition of the liquid-permeate is typically different from the chemical composition of the formula inside the package.
In this situation, the liquid-permeate typically causes corrosion of the coating (with delamination from the metal), corrosion of the underlying metal or both coating and metal corrosion. Both the formula’s chemical composition and the pH of the liquid-permeate determine if coating or metal corrosion will occur, or if both will occur together.
Some of the more common formula ingredients that typically absorb into coatings, and potentially cause both coating and metal corrosion, are:
• Water
• Emollients
• Surfactants
• Fragrances
• Formula pH
• The metal cations from organic and inorganic salts
• Organic acids
This list is by no means an exhaustive one.
Formula-ingredient absorption into a coating could also cause it to lose its barrier properties—either partially or completely. Loss of barrier properties often leads to coating delamination from the substrate metal and/or corrosion of the metal under the coating.
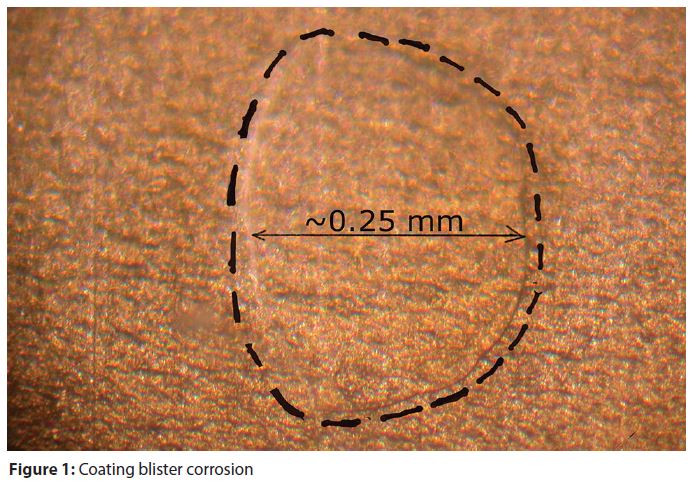
Coating corrosion could be either localized—such as blisters (Figure 1) or general, widespread delamination (Figure 2). Package metal corrosion could also either be localized under blisters or under wide areas of a coating. Metal corrosion also typically causes and/or accelerates the delamination of a coating.
Absorption of formula ingredients also lowers a coating’s glass Tg. Discussion of how Tg is changed by absorption, plus how increasing temperature affects a coating’s Tg.
We will complete this three-part series in the next issue. We’ll also discuss how raising temperature affects coating performance as a barrier and sometimes produces metal corrosion that does not occur at room temperature.