Written on: March 1, 2021 by W. Stephen Tait
Hello, Everyone. There are times when one or more formula ingredients are not soluble in the rest of the formula. In these instances, the insoluble ingredients are dissolved in another solvent before mixing with the main formula.
However, what happens when the solvent for the insoluble formula ingredient(s) is also insoluble in the main formula solvent? Enter the emulsion!
What is an emulsion?
An emulsion is a type of colloid formed from liquid droplets dispersed in a continuous liquid matrix. The dispersed droplets can have an average size of 1–1,000 nanometers (nm)—size is referred to as an average because there is typically a distribution of droplet sizes. Spray packaging dispenses liquid droplets into air; this type of colloid is referred to as an aerosol. Aerosol droplet sizes from consumer packaged goods range from approximately 4–10 microns (micro-meters) and medical aerosol droplet sizes are typically smaller than four microns. In other words, aerosol sprays are colloids but not emulsions.
Probably one of the most familiar emulsions is oil-invinegar salad dressing. This emulsion is formed by vigorously shaking a mixture of oil and vinegar to produce a translucent emulsion that immediately separates (breaks) into oil and vinegar when the shaking is stopped. In other words, oil-in-vinegar emulsions are not stable.

Oil-and-vinegar emulsions immediately separate and are not stable.
Milk is another common opaque emulsion that consists of water and small water-insoluble liquid-fat droplets. Milk also separates into two or more translucent and opaque phases over time (referred to as cream phases). However, milk also has natural surfactants/emulsifiers that stabilize the emulsion for a significantly longer time than the oil-in-vinegar emulsion.
Hence stable emulsions are formed by dispersing one insoluble liquid into another insoluble liquid with a surfactant (emulsifier). Please note that stable is a relevant term, because all emulsions eventually break (separate) into their separate insoluble components.
Types of emulsions
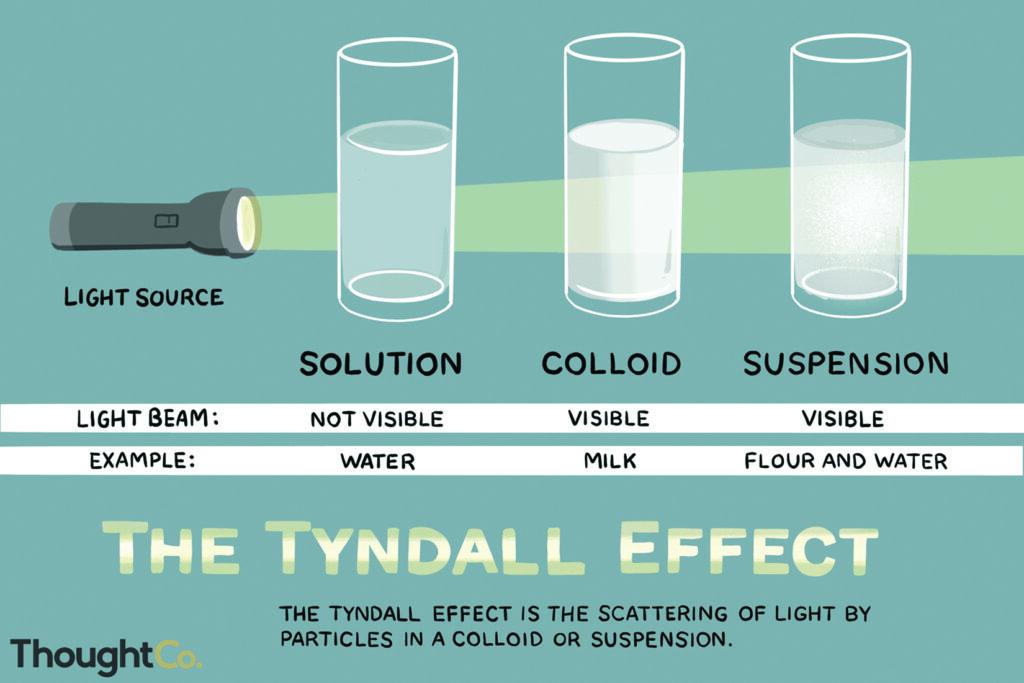
Emulsions are generically classified as either oil out—water droplets in an oil matrix, or as water out—oil droplets in an aqueous matrix. Emulsions can also be characterized by the average droplet size, concentration or by the surfactant orientation on the droplet surfaces. For example, microemulsions and nano-emulsions have average dispersed droplet sizes below 100nm. They often have a translucent, slightly blue color and a beam of light travels through the micro/nano-emulsions without being refracted by the droplets (Tyndall effect).
The dispersed phase in a hyper emulsion is above 74% of the overall formula. Hyper emulsions are typically opaque and are sometimes used to extinguish fires.
Emulsions can also be liquid crystals. In this situation, dispersed droplets are encapsulated by a crystalline sheath of surfactant molecules around the droplets.
Different surfactants for different emulsions
Surfactants are believed to stabilize dispersed droplets by either forming a protective complex liquid barrier around each droplet or by forming a charged surface around the droplets. Both barriers and charged surfaces prevent droplets from contacting other droplets and coalescing into larger droplets.
Consequently, different types of surfactants form and stabilize different types of emulsions. Indeed, there are so many different types of surfactants that I’m not even going to attempt to provide an overview of which surfactants are used for each emulsion type.
In some instances, a critical surfactant concentration is needed to form and stabilize an emulsion. Surfactant concentration can also affect emulsion droplet size and droplet concentration. For example, more surfactant is needed to form and stabilize a nanoemulsion than a microemulsion.
Emulsions corrosivity & sprays
Are emulsions corrosive toward spray package materials? The short answer is it depends! Whether or not corrosion will degrade the package materials is a function of the:
• Type of package materials
• Chemical composition of your formula
• Emulsion type
• Stability of the emulsion; and
• Corrosivity of the various phases formed from a broken emulsion
Smaller droplets coalesce into larger ones when an emulsion is unstable or destabilized. For example, a surfactant can become partitioned between the propellant and droplets at the propellant-bulk formula interface when a surfactant is soluble or partially soluble in a propellant. Partitioning decreases the surfactant concentration on the droplets, allowing them to coalesce. The larger droplets could subsequently contribute to or cause corrosion:
• In the interfacial area
• Where larger droplets stick to the interior package surfaces
• At the bottom of the package when larger droplets sink to the bottom
How fast an emulsion corrodes the package materials (i.e., the corrosion rate) determines the package service lifetime. Whether or not corrosion will occur, as well as the package corrosion rate, are both determined by:
• Emulsion type
• Chemical composition of the matrix and the dispersed droplets
• Corrosivity of the various phases resulting from a broken emulsion—corrosive phases could be free-water and/or cream-phases
• Type of surfactant used to stabilize the emulsion
Microemulsions and nano-emulsions in some instances are significantly more corrosive than emulsions having droplet sizes greater than 100nm. Increased corrosivity by micro/nano-emulsions is believed to be caused by the significantly higher surface area of the smaller droplets for these emulsion types.
Some surfactants—such as sodium lauryl sulfate, nonylphenol and di-nonylphenol—contribute to or cause spray package material corrosion.
Corrosion testing of the complete product (formula plus propellant) is recommended for emulsion spray products. Corrosion testing can be accomplished with either a one-year storage stability test or a shorter electrochemical corrosion test using appropriate test and measurement parameters. Worstcase corrosion testing by breaking the emulsion and conducting the corrosion tests on only the free-water phase is not recommended.
Thanks for your interest and I’ll see you in April. Contact me at 608-831-2076, rustdr@pairodocspro.com or from one of our two websites pairodocspro.com or aristartec.com. SPRAY