Written on: March 1, 2023 by W. Stephen Tait
Hello, everyone. In the previous issue, I began a series on material defects in spray packaging to discuss their relationship with spray package corrosion. We began by focusing on the more common defects in aluminum aerosol containers. This month, we’ll continue with the more common material defects found in laminated foil bags and tinplated steel aerosol containers.
Laminated aluminum foil bags
Flat sheets of laminated foils are folded into a bag, the seams welded together and an aerosol valve is attached to the top of the bag. The aluminum-foil is sandwiched between layers of polymer film—typically one layer on the foil’s exterior and two layers on the interior. The laminated bags are inserted into traditional aerosol containers and the product is filled into the bag.
Figure 1 has a typical example of a micro-bulge in a laminated bag. Bulges are typically small, thin and transparent, as seen in Figure 1. The micro-bulge in Figure 1 is in the interior bi-layer film (nylon on top of polypropylene). Hence, the bulge might be the result of one layer delaminating from the other. However, the actual cause for micro-bulges is unknown at this time.
It has been our experience that micro-bulges do not contribute to or cause laminated aluminum foil bag corrosion.
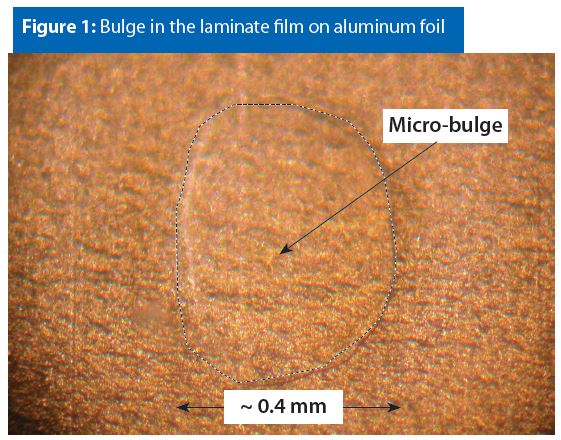
Figure 2 provides an example of a laminate polymer film that is delaminated at the bag welds. It has been our experience that this type of material defect is rare and can be avoided with good welding practices.
We have not observed instances where this type of material defect contributes to or causes spray package bag corrosion. However, this type of defect could cause bag-rupture around the delaminated area if the weld is weak.
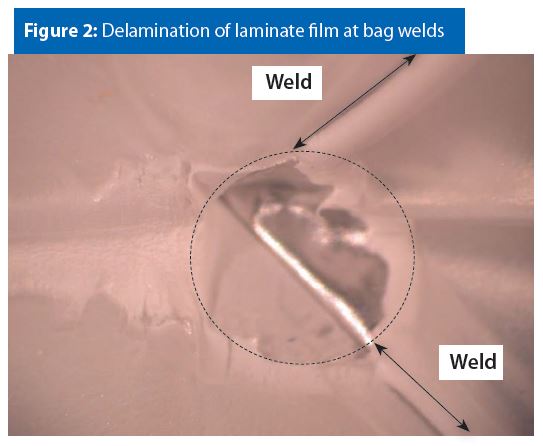
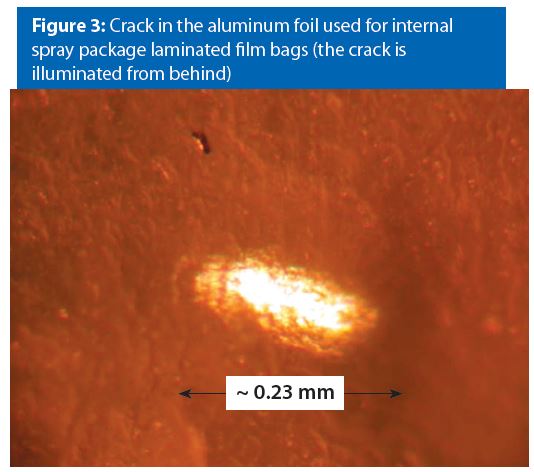
Figure 3 provides an example of a crack in the aluminum foil under the laminate film. In our experience, this type of material defect is typically associated with a single manufacturing batch. We have not observed instances where this type of material defect contributes to or causes laminate-foil bag corrosion.
It is possible that a bag with this type of defect might leak product. However, the product would have to diffuse through both the inner and outer film layers to cause a bag to leak. We have not yet observed instances where this type of defect causes bag leaking.
Tinplated steel aerosol containers
Tinplated steel (tinplate or electrolytic tinplate [ETP]) aerosol containers are available both with and without an internal polymer coating. The same coating defects discussed in last issue’s Corrosion Corner for aluminum aerosol containers are also found in coated tinplate aerosol containers. Hence, I will not repeat last issue’s discussion and will focus on unlined tinplate defects.
Figure 4 provides an example of an area where the tin coating did not cover (wet) the steel substrate. Non-wetting produces holes in the tin coating that expose the substrate steel, and/or a very thin iron-tin alloy layer—sometimes referred to as K-plate. Multiple holes in tin coatings—such as that in Figure 4—are frequently present in tinplated steel aerosol containers.

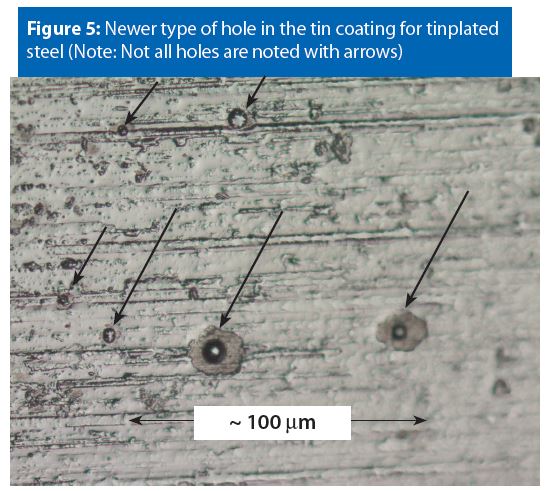
There are two different morphologies for the holes in tin coatings. Figure 4 shows the traditional type of hole morphology and Figure 5 shows a newer type of hole morphology that has appeared within the last two decades.
Notice that, when comparing Figure 4 and Figure 5, the newer holes are more symmetrical than the traditional holes. Most likely, the symmetrical newer holes arise from either a new plating bath chemical composition, a new tinplating process or variations in plating bath chemical composition.
Holes in tin coatings are potential sites for pitting corrosion. However, the chemical composition of your formula determines if holes in tin coatings will or will not result in pitting corrosion.
It is unknown at this time if the traditional hole morphology (Figure 4) or the newer hole morphology (Figure 5) result in different susceptibilities to and/or magnitudes of pitting corrosion. Corrosion testing is the only way to determine if a given formula will cause pitting corrosion at holes in the tin coating.
Steel aerosol container welds are diffusion welds formed with heat and pressure. The heat is produced from an electrical current flowing between the overlapping edges of the tinplated steel sheet used to form the cylindrical container body.
A small amount of steel metal could be ejected from under the overlapping edges when the combination of pressure and heat is not optimum. Figure 6 has an example of a small particle of metal ejected from a weld. This phenomenon is referred to as weld spatter.
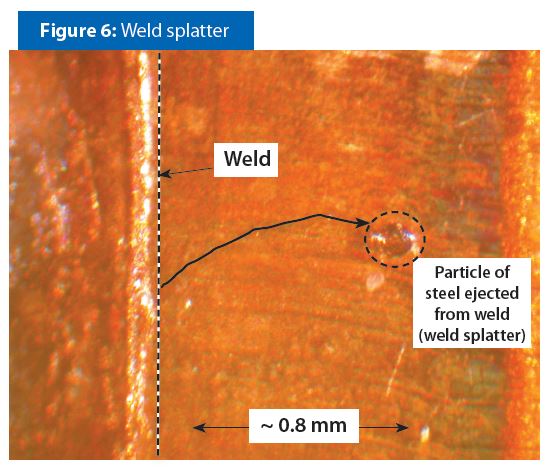 Weld spatter is not common. However, pitting corrosion could occur if weld spatter produces a small cavern in the weld. The chemical composition of a formula determines if pitting corrosion will occur in a cavern formed by weld splatter.
Weld spatter is not common. However, pitting corrosion could occur if weld spatter produces a small cavern in the weld. The chemical composition of a formula determines if pitting corrosion will occur in a cavern formed by weld splatter.
The material defect examples in this and last issue’s Corrosion Corner both provided examples of macro-defects that can be seen either with the unaided eye or a light microscope. These types of defects typically do not cause corrosion. However, there are formulas that will cause or contribute to spray package corrosion at these defects. Thus, corrosion testing is necessary for both new products and existing-product derivatives to prevent unexpected corrosion.
Thanks for your interest and I’ll see you in April. Contact me at 608-831-2076; rustdr@pairodocspro.com or from our two websites: pairodocspro.com and aristartec.com. SPRAY